Hydrogen Fuel Cells: How Halogen Adsorption Limits Nickel Catalyst Performance
October 22, 2025Ever wondered why nickel-based catalysts in your alkaline fuel cells—crucial for green hydrogen economies and sustainable energy futures—don’t perform like the high-end noble metals? Believe it or not, unassuming halogen ions in your electrolyte could be the sneaky troublemakers, hogging active sites and throttling performance. These tiny villains pack a surprisingly powerful punch.
The Halogen Factor
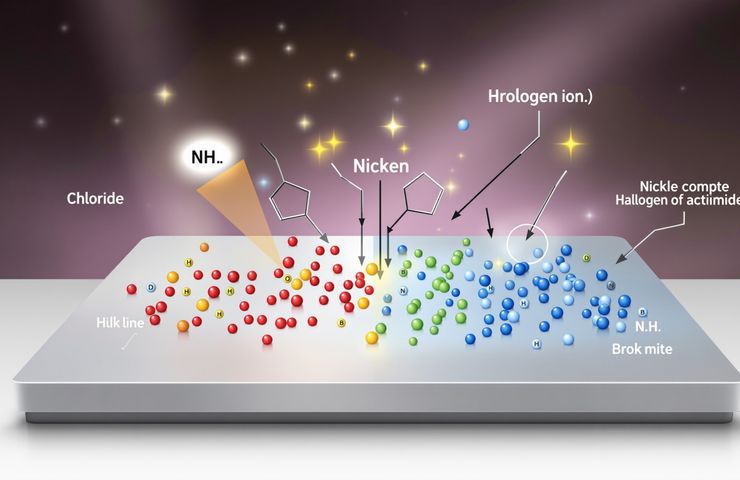
A new study in Smll, out now on Wiley Online Library, shines a harsh light on competitive halogen adsorption. Chloride, bromide and their cousins aren’t just drifting in the alkaline soup—they’re locking down the exact spots where hydrogen wants to split into protons and electrons. Under those conditions, catalytic activity can take a serious hit, especially when every fraction of a percent counts.
But wait, there’s more. Once those anions call dibs on surface sites, they can kick off corrosive pathways across the nickel. Over time, repeated cycles erode both short-term performance and long-term durability. It’s a one-two punch: instant activity loss and creeping material decay.
Why Nickel?
For ages, platinum-group metals have owned the spotlight in the hydrogen oxidation reaction (HOR), especially in alkaline conditions. But let’s face it—platinum and its pals come with a hefty price tag and tight supply. Enter nickel: abundant, budget-friendly, and a top pick for lower-cost fuel cell technology.
The catch? Nickel only manages about 1–2 percent of the HOR activity that platinum or palladium delivers under similar settings. Add halogen adsorption into the mix, and you’ve got catalysts that need a serious tune-up before they can make a run at noble metals.
Platinum vs. Nickel
What gives platinum its edge is its finely tuned electron structure—it grips hydrogen just enough to split it, but not so tightly that reaction products get stuck. Nickel, in contrast, isn’t so picky in alkaline media, leaving it open for those anion “hijackers” to camp out on its active sites. The result? Higher overpotentials and lower current densities when you stack up nickel-based electrodes, especially under real-world loads. That performance gap has sent researchers hunting for ways to supercharge nickel’s activity.
Decoding Surface Science
The team teamed up electrochemical testing with density functional theory simulations to map exactly where halogen ions like to park themselves on various nickel facets. Pinpointing these hotspots gives engineers a playbook for targeted surface protection.
Armed with this map, innovators can explore strategies—think selective alloying or nanostructured coatings—that let hydrogen have free rein while keeping halogens at bay.
Implications for Industry
If you’re crafting the next wave of hydrogen fuel cells, this study is more than just academic fluff. Cutting down on halogen poisoning could narrow the performance chasm between nickel and platinum, paving the way for cheaper catalyst layers that still hit the power density marks for everything from portable generators to heavy-duty transport.
Lower-cost catalysts translate into reduced capital costs per kilowatt and fewer stack replacements, giving fuel cell technology a stronger edge over batteries and combustion engines. In a future where every dollar counts, these molecular insights could crack open new markets and accelerate the adoption of zero-emission technology.
Environmental & Supply Chain Considerations
Cranking up production of nickel-based catalysts might ease the geopolitical and environmental strains tied to platinum mining. But remember, nickel mining isn’t magically impact-free. Tailings management, water use and community impacts all need tight sustainability guardrails.
Recycling spent nickel catalysts could fuel a closed-loop economy—recovering precious metals and trimming waste. That conversation between industry players and regulators is just getting started.
On the policy side, governments crafting critical minerals strategies can boost nickel recycling programs and green mining standards, ensuring these technologies align with broader zero-emission technology goals.
Engineering Around Halogens
One promising angle is surface functionalization—imagine a nonstick coating for your cookware. By grafting ultrathin organic films or mixed-metal oxides onto nickel, you can build an anion-repellent barrier that still lets hydrogen do its thing.
Similar protective layers have already upped chloride tolerance in platinum-based chlor-alkali electrolysis. Translating those lessons to nickel means tweaking the layer’s thickness and composition to suit nickel’s chemical vibe.
Designing Electrolytes
The electrolyte itself is another tuning knob. Swapping between potassium or sodium hydroxide, adjusting pH levels, or tossing in molecular inhibitors can keep free halogen ions busy in solution—kind of like seasoning a stew: a little tweak in the mix can shift the whole flavor profile.
Some labs have experimented with phosphate or borate additives that selectively corral halogens, keeping them away from the catalyst surface. Early results show modest activity and stability gains, but the perfect additive cocktail is still under exploration.
Potential for Scale-Up
Lab-scale setups tell only part of the story. Real-world stacks face heat swings, pressure changes and impure feedstocks. The next step? Pilot tests that prove these anti-halogen tactics can hold their own under harsh conditions, maintaining steady performance over thousands of hours.
Accelerated stress tests—simulating repeated start-stop cycles—will be crucial to see how well anion-blocking coatings stand up to the long haul. That durability check could make or break commercial possibilities.
A Catalyst for Collaboration
These findings underscore the magic of cross-disciplinary teams. Surface chemists, computational theorists and electrochemical engineers all pitched in to crack a problem that none could tackle solo. As labs and companies share data, co-innovation platforms can speed up discovery cycles, bringing low-cost, high-performance nickel catalysts to market faster.
Public-private partnerships and open-science initiatives might then zero in on standardized test protocols for halogen poisoning, ensuring breakthroughs in one lab translate smoothly to industry-wide wins across the hydrogen fuel cells value chain.
Looking Ahead
It’s a good reminder that sometimes the smallest players—mere ions—make the biggest difference. By demystifying how halogen adsorption trips up alkaline hydrogen oxidation, the team at Wiley Online Library isn’t just unraveling surface science—they’re pointing the way toward affordable, green hydrogen solutions.
As we push for scale and commercialization, these molecular insights could serve as the blueprint for a more accessible, zero-emission technology era, bringing truly clean energy closer than ever.



 With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.
With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.