Hydrogen Production from Seawater Achieved with Liquid Gallium Process
February 11, 2026When you think about hydrogen production, your mind probably jumps to fancy electrolysers and truckloads of purified water. But what if I told you the world’s most plentiful liquid—seawater—plus a healthy dose of sunlight could do the trick? That’s exactly the clever shortcut cooked up by the team at University of Sydney. On February 10, 2026, PhD candidate Luis Campos and senior researcher Professor Kourosh Kalantar-Zadeh rolled out a process that uses floating liquid gallium particles in seawater to whip up green hydrogen at an impressive 12.9% efficiency—no extra electricity, no fancy water purification needed.
It’s a neat two-for-one fix, tackling both freshwater shortages and energy-hungry production head-on, thanks to a mash-up of materials chemistry and good old sunshine. As Campos puts it, “We’ve unlocked a way to pull sustainable hydrogen straight out of the ocean, powered purely by light.” Their findings, fresh off the press in Nature Communications, lay out a circular setup where gallium oxidizes, is chemically refreshed, and then dives back into action, kicking out hydrogen in a never-ending loop.
A New Approach to Green Hydrogen
Most folks aiming for green hydrogen stick to membrane-based electrolysis—think ultra-pure water, hefty electrical inputs, and a complex infrastructure. Others flirt with photocatalysts or plasma splitting, but those still demand a lot of prep or juice from the grid. The Sydney squad has taken a different path by leaning on liquid gallium’s low melting point and broad solar absorption. By sprinkling micrometre-sized gallium droplets into seawater, they create a light-sensitive cocktail that, when sun hits it, splits water molecules and sends H₂ gas flying off, turning the gallium into gallium oxyhydroxide in the process. Hitting nearly 13% solar-to-hydrogen conversion at the lab bench isn’t shabby—it’s on par with early milestones in solar-cell history.
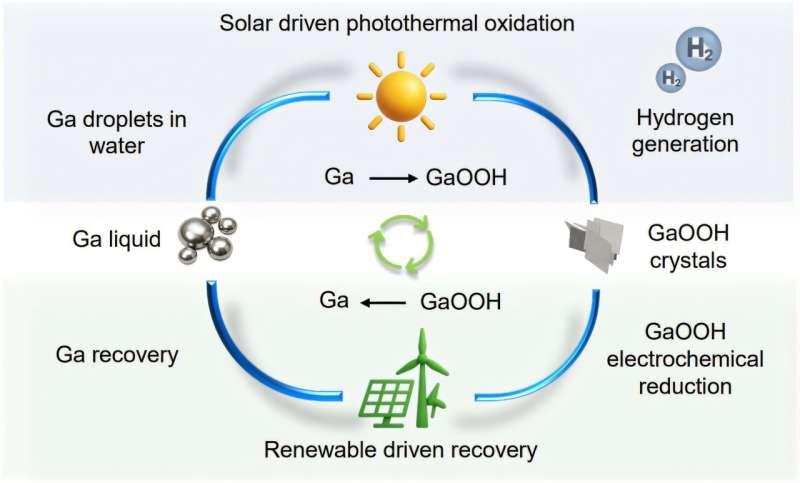
How the Liquid Gallium Process Works
At its heart, the trick relies on two star qualities of liquid gallium: it stays fluid at room temperature and soaks up sunlight across a broad spectrum. Picture a shallow reactor filled with seawater and suspended gallium droplets. Sunlight bathes the mixture, the gallium surface oxidizes, and water molecules split apart, popping out hydrogen gas. The leftover gallium oxyhydroxide is scooped up, treated with a mild chemical reducer, and bam—the metal springs back to life, ready for the next cycle. Because you’re not ditching the gallium each run, it can loop through hundreds of stages with barely any loss, unlike one-and-done catalysts.
Learning from Past Technologies
Hydrogen production has leaned on electrolysis since the early 1900s, and more recent efforts have chased photocatalysts that mimic how plants harvest sunlight. Progress has been solid but slow, stymied by the need for pure water, pricey metal catalysts, and big energy inputs. The Sydney project echoes the early days of photovoltaic cells—those first solar panels kicked off at about 6% efficiency in the 1950s, then breezed past 10% within a couple decades. Topping 12.9% already signals that, with extra tinkering on materials and reactor design, this gallium-based approach could scale up even higher.
Path to Commercialization
The University of Sydney has locked down a patent covering everything from reactor blueprints to the gallium recovery trick and process flow. Backed by the Australian Research Council Discovery Project, the researchers are moving out of the lab and into mid-scale photochemical reactor design. Next up: dialing in droplet size, tweaking reactor shape, and concentrating sunlight just right to crank up output. Dr. Francois Allioux, co-lead on the project, reckons that ditching pumps for water purification and skipping high-voltage gear could slash both capital and operating costs—putting this seawater hydrogen tech in the same ballpark as traditional electrolyser systems.
Implications for the Hydrogen Economy
In a world sprinting toward net-zero, tapping into seawater hydrogen could be a total game-changer. Australia, with its massive coastline and big export ambitions, stands to gain from a setup that pairs abundant marine resources with solar power. Coastal plants could churn out hydrogen without draining freshwater reserves or overloading the grid, supplying zero-emission fuel for next-gen fuel cell technology, ammonia production, and heavy industry decarbonization. Skipping the need for ultra-pure water means even remote or arid locales could hop on the hydrogen bandwagon, reshaping global supply chains for the better.
Challenges and Next Steps
Of course, no breakthrough comes without hurdles. Scaling up liquid gallium to industrial levels means locking in steady supply chains and keeping costs in check—gallium isn’t quite as common as aluminium or iron. Reactor designs will have to handle the quirks of real-world seawater—salinity swings, temperature changes, biofouling—while still churning out consistent hydrogen yields. And before anyone invests big, we’ll need full life-cycle analyses to prove this route really does beat the carbon footprint of existing methods. The team’s already crunching numbers on these variables as they map out their commercialization roadmap.
Looking ahead, Professor Kalantar-Zadeh is aiming to get a prototype pilot plant up and running within three years, with an eye on pushing solar-to-hydrogen efficiency past 15%. If all goes to plan, weaving liquid gallium reactors into coastal energy hubs could kick off a new chapter in sustainable energy, where seawater and sunlight become the dynamic duo powering a clean hydrogen future.
For now, the University of Sydney’s proof-of-concept is a powerful reminder that sometimes the simplest ingredients—seawater and a drop of liquid metal—can unlock the boldest paths to zero-emission goals.
source: techxplore.com



 With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.
With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.