Palladium Membrane Breakthrough Boosts High-Temperature Hydrogen Separation
October 13, 2025Way back on October 1, 2025, MIT engineers quietly dropped a bombshell in the clean energy community: they’ve developed a palladium membrane that can pull hydrogen out of gas mixtures at temperatures above 1,000 K—blowing past the old 800 K barrier. This leap in high-temperature hydrogen purification could be a real game-changer, making clean hydrogen fuel systems more compact and ditching those energy-sapping chillers once and for all.
The appeal of hydrogen is huge: burn it or feed it into a fuel cell, and the only thing that comes out is water vapor. From steel mills to power plants, everybody’s eyeing it for major carbon cuts. But there’s always been that nagging hitch—how to handle hydrogen separation at high heat. Traditional palladium membranes demand you cool down the gas before purification, piling on extra gear and power costs.
Now, palladium membranes themselves aren’t exactly brand-new—they’ve quietly powered semiconductor fabs and food processing for years, delivering pure hydrogen when you need it. Trouble is, crank the temperature past roughly 800 K and those palladium islands start to clump, the film fractures, and performance tanks. Nailing down that thermal stability? It’s basically the holy grail for anyone in membrane research.
Why Does High-Temperature Separation Matter?
Sure, jumping from 800 K to beyond 1,000 K might sound like a small dial twist—but in reality, it’s huge. Processes like steam methane reforming run hot—around 900–1,000 K—to crack natural gas into hydrogen and carbon monoxide. Likewise, ammonia cracking goes high-temp to split ammonia back into hydrogen. A membrane built to survive those conditions means you can perform high-temperature hydrogen purification right in the hot zone. No cooling detours, no efficiency penalties.
Inside the Palladium Plug Membrane
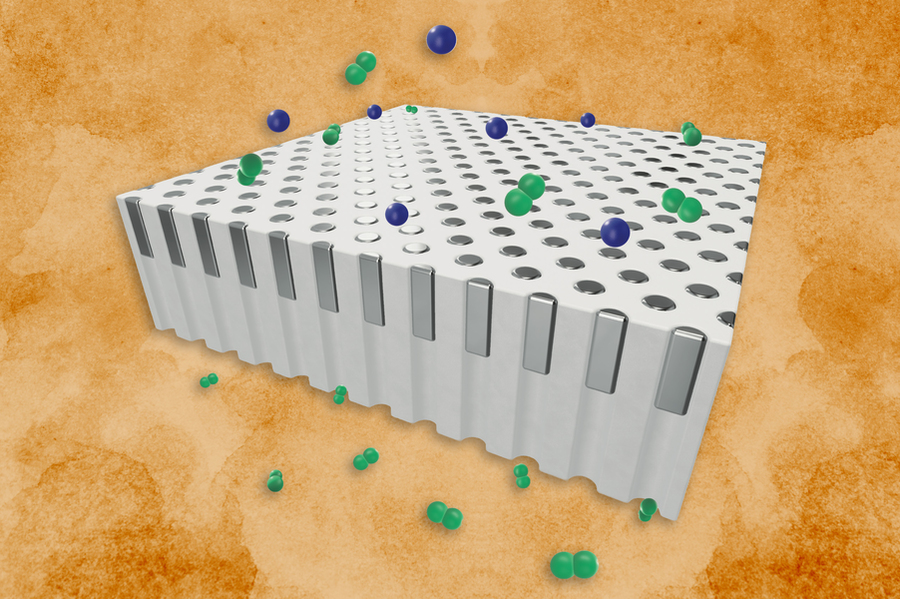
So, what’s the secret sauce? Instead of laying down a continuous palladium film, the MIT crew filled the pores of a silica support with nanoscale “plugs” of metal. When hydrogen molecules strike a plug, they split into atoms, diffuse through the lattice, then recombine on the flip side. Those little plugs stay put even up to around 1,000 K, dodging the agglomeration and dewetting issues that torpedo conventional films. What you end up with is steady hydrogen separation and ironclad structural integrity—no cooling loops needed.
“By confining palladium into discrete plugs, we’ve unlocked stability at temperatures that were previously thought impossible,” says one senior engineer on the project. “That opens the door to more streamlined, efficient hydrogen plants.”
Who’s Behind the Innovation?
This breakthrough comes straight from the heart of MIT’s School of Engineering, a place that has a long history of pulling lab ideas into the real world. While they haven’t spotlighted individual names, the team worked hand-in-glove with industry and government players—true to MIT hydrogen technology’s collaborative spirit. Their peer-reviewed results landed in Advanced Functional Materials, adding weight under every lab coat.
MIT’s track record in energy research is legendary, from early fuel cell prototypes to cutting-edge battery and solar advances. This latest plug membrane design builds on decades of deep materials science know-how, showing once again why they’re at the forefront of turning theory into industrial impact.
What’s in It for Industry?
For companies hunting leaner, greener operations, here’s the skinny:
- Avoiding pre-cooling: bye-bye bulky chillers and big capital expenses.
- Smaller equipment footprints: slip these membranes right into hot reactors.
- Lower palladium usage: the plug structure pools metal exactly where you need it.
- Faster throughput: hydrogen comes off the membrane ready to go, no thermal detours.
- Fusion-ready separation: ideal for splitting tritium and deuterium in next-gen reactors.
With fewer cooling loops, you also cut a chunk of the energy penalty—critical as everyone pushes for clean hydrogen fuel and tighter emissions goals.
Where Might We See This Next?
Today, the plug membrane is still in an MIT lab, but the roadmap is clear: scale up the fabrication, run stress tests with real industrial gas mixes, and lock in partnerships with plant operators. While there aren’t any formal commercial deals on the table just yet, you can bet pilot programs will pop up in the next couple of years if everything goes to plan.
As electrolyzer rollouts ramp up and global hydrogen hubs start to form, membranes that can handle the heat will be a linchpin for delivering reliable, low-carbon hydrogen. Keep your eyes peeled—innovations in palladium membrane tech and next-level hydrogen separation might just redefine how we power everything from factories to fuel-cell vehicles.
source: MIT



 With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.
With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.