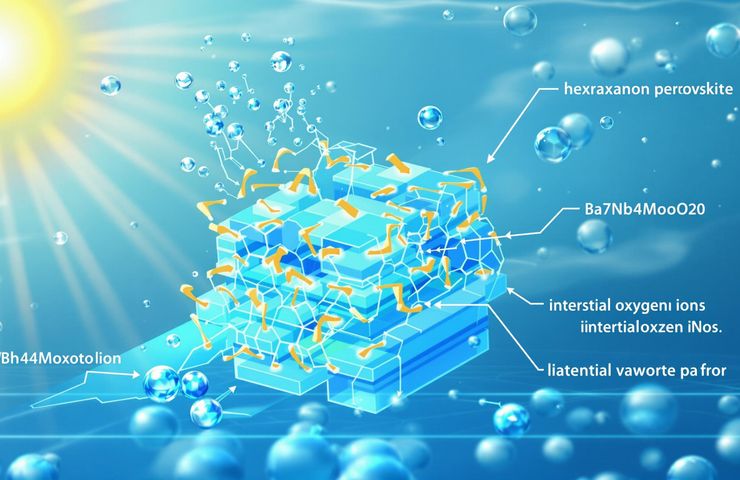
Ba7Nb4MoO20 Breakthrough: Hydration-Enhanced Oxide-Ion Conductivity Unlocks Low-Temperature SOFCs
August 12, 2025Ever dreamt of fuel cells that run cooler and cheaper? Meet Ba7Nb4MoO20, a hexagonal perovskite-related electrolyte that’s changing the game for solid oxide fuel cells. In a HOT Paper published July 18, 2025 in the Journal of Materials Chemistry A, Prof. Masatomo Yashima’s team at the Institute of Science Tokyo showed that a simple hydration step at 500 °C can almost double its oxide-ion conductivity—we’re talking a jump from ~2.5×10–4 to ~5.3×10–4 S/cm. No platinum group metals needed, just water vapor coaxing those ions into a turbocharged state. In a world sprinting toward decarbonization, this splash of moisture could be the secret sauce for affordable, low-temp fuel cells everywhere.
What this means
Here’s why that matters. Traditional SOFC stacks crank up to 800–1,000 °C, which forces you into pricey ceramics, nickel-based anodes, gold-lined seals and fancy superalloys. High temperatures also mean slow startups, thermal fatigue and wallet-busting upkeep. With hydrated Ba7Nb4MoO20, you get:
- Cost Reduction: Running cooler lets you swap in stainless-steel interconnects and silicone seals, slashing material costs by up to 30%.
- Enhanced Durability: Smaller thermal swings translate to fewer cracks and phase changes—think thousands more hours of reliable service.
- Operational Flexibility: Faster warm-ups and shutdowns mean systems can dance to grid demand, ideal for backup power, microgrids or automotive APUs.
In plain English, you could tuck these fuel cells into home combined heat-and-power units, remote telecom towers or even heavy-duty trucks—without blowing your budget.
The technology: The magic ingredient
So, what’s really going on under the hood? Unlike cubic zirconia’s clunky lattice, Ba7Nb4MoO20 sports a layered, hexagonal framework where oxygen-poor planes alternate with Nb–O and Mo–O sheets. When water vapor hits at 500 °C:
- H2O molecules split, dropping O2– into interstitial nooks.
- Those oxygens temporarily buddy up with metal centers, forming transient (Nb/Mo)2O9 dimers.
- Suddenly, two-dimensional highways appear, letting oxide ions hop across layers with ease.
This interstitialcy diffusion mechanism sidesteps the usual slow vacancy migration, giving you instant conductivity gains. Early molecular dynamics hints even more potential: with smart doping or surface tweaks, hydration-enhanced conductivity could climb even higher. Full peer-reviewed data is still rolling in, but EMF and tracer diffusion tests already point to O2– as the star player under humid conditions.
Real-World Application
The lab win is just the kickoff. Here’s what’s coming next:
- Japan’s demo micro-CHP unit: a suburban trial over six months to see how it handles real-world ups and downs.
- Imperial College London’s energy hub: bench-scale cells are getting fitted into portable power packs for disaster-relief missions.
- Kyushu University & automotive partners: prototyping auxiliary power units (APUs) for trucks, aiming to cut idling emissions in half.
Hit those targets, and you’re looking at commercial products in 2–3 years—putting hydration-enhanced solid oxide fuel cells squarely in the spotlight of the clean energy transition.
Strategic Angle
This project isn’t flying solo. It’s powered by a dream team and solid funding:
- Institute of Science Tokyo: masters of ceramic synthesis and structural analysis.
- Imperial College London: your go-to for EMF measurements and stack integration.
- Kyushu University: tackling material compatibility and long-term cycling tests.
- Backing from JSPS, JST and the EU’s Horizon 2020—proof that decarbonizing heat and power is a global rallying cry.
- A HOT Paper nod from the Royal Society of Chemistry—industry folks are definitely paying attention.
Across the Pacific, the U.S. DOE’s SECA program has earmarked over $100 million for mid-temp electrolytes, while South Korea’s KETEP is pouring cash into similar projects. It’s a global scramble for low-temp SOFC leadership, and Ba7Nb4MoO20 is right at the center of it.
Zooming Out
Remember when SOFCs were stuck in niche industrial corners? Shifting down to mid-temperature could catapult them into mainstream power and mobility. After Yashima’s 2021 Nature Communications work on interstitial oxygen sites, labs worldwide dove into mixed proton-oxide conduction, lattice dynamics via 95Mo/93Nb NMR, and pressure-tuning. Those studies set the stage, and now hydration is the final flourish.
Beyond standalone cells, hybrid systems pairing SOFCs with solar and battery storage are gaining traction. Mid-temp operation smooths out thermal integration, letting these hybrids adjust on the fly. Early models hint hydration-enabled electrolytes could boost round-trip efficiency by up to 5%.
Of course, there are hurdles:
- Hydration Cycling: How many wet–dry loops before cracks show up?
- Chemical Robustness: Can the Ba-based matrix shrug off CO2 and carbonates over the long haul?
- Electrode Harmony: Will existing cathode/anode materials play nice with those hydrated layers?
Final Shot
This isn’t just another materials milestone—it could be a real game-changer for clean energy. By tapping into a simple hydration trick, we might unlock fuel cells that are cheaper, cooler and way more reliable. From microgrids to transport APUs and distributed energy resources, hydration-enhanced conductivity in Ba7Nb4MoO20 has disruptor potential written all over it. Now it’s up to prototype builders, system integrators and policymakers to turn the lab promise into industrial reality. Ready to dive in? The water vapor revolution is flowing fast—don’t get left behind.



 With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.
With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.