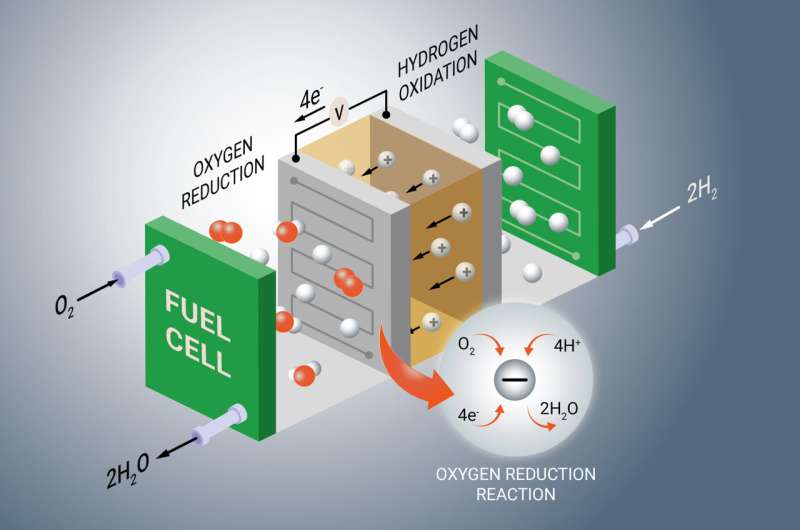
Fuel cell technology: dynamic rate-limiting steps unveiled in catalyst kinetics
January 6, 2026Just when you thought you’d got a handle on the chemistry behind hydrogen fuel cells, researchers at the Fritz Haber Institute of the Max Planck Society go and shake things up. They’ve found that the oxygen reduction reaction (ORR) in fuel cell catalysts isn’t dominated by one sluggish step like everyone assumed. Nope, it’s more like a relay race: different stages vie for the title of rate-determining step, and who’s in charge flips when you dial up the overpotential or tweak oxygen pressure.
Dr. Öner and the team have dropped their study in Nature , showing that four distinct catalyst recipes literally swap which step is the slowpoke under conditions you’d see in the real world. That overturns a decades-old electrochemistry staple—that you could squish a multi-step process into a single, easy-to-pin-down bottleneck. Instead, they’ve sketched out a fresh kinetic framework, tying how the catalyst’s surface morphs under bias to big-picture activation parameters.
Shifting the Paradigm of ORR Kinetics
Traditional models treated the oxygen reduction reaction like a rigid domino chain where one piece—often the O–O bond break—ruled the roost. But Dr. Silva and Jody Druce noticed that ramping up the extra voltage (aka overpotential) or changing oxygen pressure flips the script. Suddenly, it’s not just bond-breaking on the hook—you’re juggling electron transfer, proton coupling and surface adsorption steps, and whichever one lags behind decides the reaction’s pace.
They caught all this in real time using operando methods—a mash-up of electrochemical measurements with on-the-spot spectroscopy and microscopy. What popped out was a living, breathing catalyst surface: bias-driven reshaping, on-and-off oxidation of surface facets, shifting adsorption energies—the whole shebang feeding right back into the kinetics. No more treating catalysts like static black boxes; they’re dynamic actors tweaking their performance by the millivolt.
Prof. Dr. Beatriz Roldán Cuenya, head of the Interface Science Department, says these insights finally close the gap between quirky lab observations and the neat rate-law models theorists love. “We’d been spotting hints of bias-dependent changes for years,” she laughs, “but without a unifying kinetic story, it was all just scattered anecdotes. Now, we can actually predict how structural wobbles shift the ensemble activation energies.”
By laying out degree-of-rate-control maps across voltages and pressures, the team whipped up contour plots that spotlight which intermediate holds the crown in different zones. This multi-angle view of catalyst kinetics blows apart the old “one rate-determining step” myth, giving scientists a flexible landscape—vital for designing fuel cell catalysts that stay in their sweet spot no matter how you run them.
Why This Matters for Fuel Cell Technology
Fuel cells live or die by how slick their ORR game is: slow kinetics mean gag-worthy overpotentials, wasted juice and fattened price-tags. Realizing that the catalyst’s Achilles’ heel hops around with the operating conditions means we can target and beef up the specific steps where it stumbles—saving us from chasing a unicorn “perfect” catalyst.
Think about heavy-duty haulers or long-range electric trucks: they swing from stop-start traffic to a steady highway cruise, and a catalyst built for one mode might flatline in the other. This new kinetic map lets engineers tweak everything from surface makeup to support tweaks so the catalyst stays cruising in its efficiency sweet spot, whatever the duty cycle.
From a strategy angle, companies diving into fuel cell technology can use these findings to de-risk R&D big time. Rather than shelling out on endless lab batches, they can simulate how tossing in certain alloying pals or support oxides nudges the rate-control landscape. That means quicker prototypes, less wasted material and catalysts built for real-world grit and stability.
From Lab to Real-World Applications
Sure, the spotlight was on four catalyst blends, but this playbook is totally general. Be it platinum-based alloys for PEM fuel cells or low-cost, non-precious metal contenders, you can apply this degree-of-control analysis across the board. It’s a game-changer for electrocatalysis, whether you’re cutting CO2 emissions or juicing up water for hydrogen.
What’s more, it’s a wake-up call to dust off old data. Tons of past kinetic studies might look completely different through this dynamic-rate lens—potentially surfacing performance boosts hiding in plain sight. That means funding agencies and industrial partners have a green light to hustle follow-up studies, re-scanning promising catalysts with fresh eyes.
Regulators and standards bodies will probably sit up too. Benchmark tests built on single-step models might evolve into multi-parameter scorecards, making sure catalysts really deliver efficiency across every twist and turn of operating conditions.
At its core, this work is steering the field toward a holistic design vibe: instead of the age-old “What’s the rate-determining step?” we’re asking, “How do all these steps tango as the conditions wobble?” It’s a mindset shift that promises smarter, more robust hydrogen fuel cells—on the road, at sea, or backing up power grids.
As Dr. Öner puts it, “We’re not just fine-tuning catalyst edges—we’re redrawing the roadmap for how we understand and optimize electrocatalysts.” That new map could very well chart the course for tomorrow’s zero-emission technologies.


 With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.
With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.