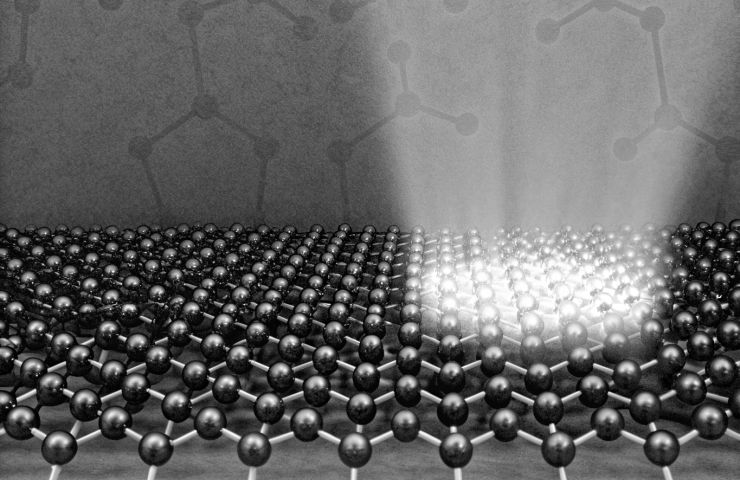
Hydrogen Production Breakthrough: Light-Driven Heterolytic H₂ Splitting at Room Temperature
September 8, 2025Ever had that daydream of using sunlight to split hydrogen molecules at room temperature? Well, the clever folks at the Dalian Institute of Chemical Physics (DICP), teamed up with the University of Trieste, have done exactly that. Under the guidance of Prof. Wang Feng and Prof. Paolo Fornasiero, they’ve come up with a photocatalytic heterolytic hydrogen dissociation trick using a Au/TiO₂ catalyst that works at ambient conditions. If you’re wondering what this means for hydrogen production and the push for sustainable energy, buckle up—it’s a big deal.
Reimagining Hydrogen Activation
We all know traditional hydrogenation is basically a furnace show—heat a nickel or platinum catalyst to anywhere between 100–300 °C, crank up the pressure to 10–50 bar, and hope for the best. That’s energy-intensive, pumps out CO₂, and doesn’t exactly scream green hydrogen. The dream of chopping H₂ into a proton and a hydride (that’s heterolytic splitting) at room temp has always been the holy grail in fine chemicals. But until now, it was more wish than reality.
Enter the Au/TiO₂ showstopper. Flash some UV light on these TiO₂ nanoparticles and boom—electrons and holes pop into existence. The electrons scamper off to little gold islands on the surface, while the holes get cozy at the Au–O–Ti junctions. This spatial charge segregation slashes the barrier you usually need heat and pressure to overcome, letting H₂ split heterolytically at room temp and one atmosphere. Spectroscopy and isotopic tests confirmed it—science, right?
Photosynthetic Pretenders
Using light to split molecules feels a bit like playing at nature’s homework—after all, plants have been doing it forever. But replicating that botanical wizardry in an industrial setting has been a slog. Early photo-bits kept recombining electrons with holes in a nanosecond, meaning yields were dismal. Scientists have flirted with Au/TiO₂ since the 1980s, but it wasn’t until this crafty interfacial engineering that the charge separation stuck long enough to actually cleave H₂ at room temperature. It’s a milestone in the march toward light-driven catalysis.
Cracking CO₂ Almost Perfectly
But the team didn’t stop at a fancy split—they fed that freshly minted H₂ straight into CO₂ hydrogenation in a fixed-bed photocatalytic reactor. And the result? Nearly 100% single-pass CO₂ conversion with over 99% selectivity for ethane. In plain speak, you shove in a CO₂–H₂ cocktail, shine some light, and out pops ethane, ready to be turned into plastics, solvents, or fuel. It sidesteps the pesky equilibrium traps of thermal methods and keeps unwanted side reactions in check. Plus, after 1,500 hours of continuous run, the catalyst was barely winded—prime for scaling up.
Why It Matters for Industry
Look, hydrogenation is the bread and butter for a quarter of the global chemical biz. But it’s also one of the toughest puzzles in industrial decarbonization, given the heat, pressure, and pricey gear you need. If you can tame H₂ activation at room temperature with a beam of light, you could slash energy consumption by 30–50%, shrink scope 1 emissions, and dodge the hazards of high-pressure hydrogen tanks. That’s a game-changer for green hydrogen adoption.
Even better, you can bolt these photo steps onto existing thermal reactors—think retrofits, not a full teardown. Imagine coal-heavy plants in northeast China flipping the switch to a hybrid photothermal system, turning waste CO₂ into value-add hydrocarbons using solar or UV-powered electricity. That’s the sort of sustainable energy pivot industrial players crave.
Policy, Investment and Market Dynamics
Governments around the world are pouring cash into green hydrogen and low-carbon technologies. In China, the Chinese Academy of Sciences has flagged photocatalysis as a priority in its five-year blueprint. Over in Europe, Horizon Europe is shoveling funds at solar-to-fuel projects. DICP and Trieste are surfing this policy wave, which means light-driven hydrogen activation could be a hot ticket at the next round of grants or private equity pitches.
Enterprise Angle
Big chemical companies—and let’s face it, oil majors too—are desperate for tech that ticks the box on net-zero goals without breaking the bank. A module based on photocatalytic heterolytic hydrogen dissociation could slot neatly into existing ammonia or methanol facilities, pumping up yields without tearing down walls. For investors, it’s a sweet spot: lower CAPEX and OPEX than old-school reactors, plus the chance to pair with solar farms for truly zero-emission technology.
Anchored in Dalian’s High-Tech Ecosystem
All this magic is brewing in Dalian, Liaoning Province—a city that’s morphed from a petrochemical stronghold into a powerhouse of R&D. DICP’s campus buzzes with projects on photocatalysis, hydrogen production, and sustainable energy. By teaming up with the University of Trieste, they’re blending local know-how with European materials chemistry savvy—a neat East–West tag team aimed at speeding discoveries from the bench to the factory floor.
From Bench to Pilot
That Au/TiO₂ catalyst isn’t just a one-trick pony. After 1,500 hours of non-stop action in lab runs, it still had fight left in it—an encouraging sign for industrial deployment. The squad even tested variants like Au/N‐TiO₂ and Au/CeO₂, hinting at a flexible platform. Sunlight-driven experiments with solar simulators nailed the same heterolytic split, setting the stage for reactors powered straight by sunlight.
To gear up for scale, the researchers are tweaking everything from reactor shape for better light coverage to gold nanoparticle size for optimal electron trapping. They’re also scouting co-catalyst dopants to sharpen selectivity. Rumor has it pilot plants could fire up as early as 2027, with DICP courting industry partners to speed up commercialization.
Expanding the Scope
While the initial demo zeroed in on CO₂-to-ethane, this light-powered heterolysis could crack open routes to ethylene, propylene, or even ammonia—no furnace required. Early tests show over 95% selectivity for ethylene with minor tweaks to the catalyst. And by mixing in plasmonic metals like silver or fine-tuning the Au/TiO₂ interface, they’re inching toward visible-light activation, which means less reliance on UV lamps and more on natural sunbeams. Talk about building out robust hydrogen infrastructure.
No breakthrough is flawless. Right now, you need UV LEDs or lamps to jump-start TiO₂, which adds to energy costs. But with doped TiO₂ and new semiconductors on the horizon, visible-light photocatalysis might be around the corner. Cost analyses suggest that, even factoring in the extra juice for UV, the overall energy savings from room-temp operation still tips the scales. Engineering compact, high-intensity light reactors will be key to getting this into the field pronto.
Next Steps Toward the Sun
Looking ahead, the team is mapping out how to plug this tech into electrolyzers and fuel cell stacks, dreaming of a closed-loop setup where hydrogen storage merges seamlessly with light-driven conversion. If they can push solar-to-fuel efficiencies past 5%, we might see commercial rollouts sooner than you think. And who knows? In a few years, modular light-driven hydrogenation units could pepper petrochemical parks worldwide, turning nothing but sunbeams and CO₂ into the chemicals we all depend on.
It’s early days, sure, but the breakthrough from DICP and the University of Trieste is a vivid reminder that sometimes the simplest idea—splitting H₂ with light—can cast the brightest beacon for a hydrogen infrastructure revolution.


 With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.
With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.