Hydrogen Production: How Catalyst Interfaces Drive Oxygen Evolution Reaction Efficiency
September 4, 2025Ever paused to think why electrolysis needs so much oomph just to split water into hydrogen and oxygen? It all comes down to one stubborn step: the oxygen evolution reaction (OER). After years of catalyst tinkering, OER still sits squarely as the speed bump in green hydrogen production. As we chase cleaner fuels, every watt in hydrogen production counts—green hydrogen is poised to be a lynchpin for sustainable energy and industrial decarbonization.
Identifying the Molecular Switch
Right at the core of the OER is a bias-dependent tipping point where the catalyst suddenly shifts from dragging its feet to firing on all cylinders—and that’s where the magic happens. Under the leadership of Dr. Martinez-Hincapié and Dr. Oener, guided by Prof. Beatriz Roldán Cuenya at the Department of Interface Science, the team leveraged a combo of operando X-ray spectroscopy and temperature-dependent electrochemistry across a suite of voltages and temperatures. Their results, published in Nature, reveal that once you clear a specific overpotential threshold, the catalyst surface sheds accumulated charges and jolts into a high-activity mode—no bells or whistles, just pure reaction kinetics taking off. This breakthrough has big implications for tuning catalyst design in real-world electrolyzers pushing for carbon cuts in industrial decarbonization.
The kicker? It isn’t about loading more catalyst onto the electrode. It’s all about the tiny world right where the solid catalyst meets the liquid electrolyte.
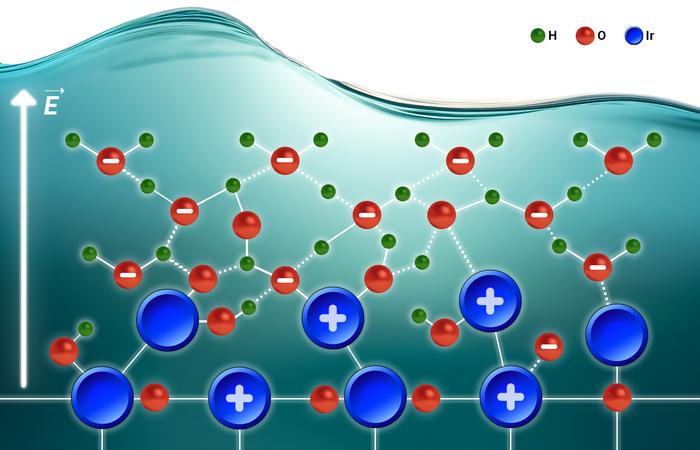
Diving into Interfacial Solvation
Picture that interface as a micro-thin layer where water molecules, ions, and catalyst atoms are constantly mingling. This solvation film helps prep the OER’s four-electron “dance,” smoothing the path for oxygen to break free. By cranking up the temperature in controlled steps, the researchers watched how charge flow and ion arrangement evolved. What they found is eye-opening: intrinsic activity leans more on interfacial solvation than on how much catalyst you’ve crammed on or how large its surface is. Tweaking electrolyte composition—like swapping in different buffers—can reshape this solvation film, offering a fresh lever to optimize hydrogen production efficiency.
In everyday terms, it’s not about heaping on extra catalyst powder—it’s about how water and ions cozy up around each active site. This flips the script from bulk material tweaks to the nitty-gritty of surface chemistry.
Operando X-ray Spectroscopy in Action
With real-time X-ray absorption and emission techniques, the team literally watched the catalyst’s oxidation state shift the moment they nudged the voltage past that sweet spot. Think of this operando analysis as a molecular dashboard, showing charge build-up and structural twists as they happen. These operando snapshots also shed light on fleeting intermediates that dictate long-term stability, helping chemists design catalysts that don’t just sing at first but stay in tune after thousands of cycles—key for robust sustainable energy systems. Without this real-time view, those short-lived states would vanish faster than you could say “green hydrogen.”
Temperature-Dependent Electrochemistry: A Deeper Dive
Then, by dialing the temperature up and down, they mapped how solvation dynamics tweak the reaction speed. Warmer conditions boosted ion mobility, sharpening the line between a charge-limited crawl and full-throttle activity. It’s like tuning your radio: find the right temperature, and the OER signal comes in crystal clear. Plus, by mimicking a range of operating temperatures—from chilly startup settings to hotter industrial streams—they can predict how these catalysts will behave in real electrolyzer stacks, whether deep in a lab or out under the sun.
A New Paradigm in Interface Engineering
For ages, catalyst designers poured their energy into cranking up surface area or fiddling with bulk composition. Now, the spotlight’s on that invisible layer of water and ions hugging each active site. Fine-tuning this nanoscale microenvironment doesn’t just boost OER; it could also harmonize with the hydrogen evolution reaction (HER), potentially unlocking more efficient two-sided electrolyzer designs.
Implications for Catalyst Design
So, what’s the takeaway for the real world? For starters, electrolyzer makers can shift their focus from turbocharging surface area to mastering catalyst-electrolyte interactions. That tweak alone could slash material costs—no need to chase pricey metals. By zeroing in on interfacial details, designers might stumble upon catalysts made from earth-abundant materials like manganese or iron oxide, rather than relying heavily on scarce iridium or ruthenium.
Even more exciting: the transition potential didn’t budge with changes in catalyst loading or surface area—a fact the team verified experimentally. This hints at a universal rule of thumb: nail the interfacial conditions, and OER performance becomes a whole lot less finicky about bulk properties.
Shifting Gears from Bulk to Interface
For years, the electrolysis conversation revolved around cranking up high-surface-area foams and nanostructures. But as computational tools and vibrational spectroscopies got sharper, researchers realized the true battlefield lies in that solvation shell. Coupling these insights with machine learning and advanced simulations means we can predict the best candidate interfaces before even making them, accelerating the push toward cost-effective green hydrogen platforms.
From Lab to Market: Next Steps
Of course, weaving these interface-engineered catalysts into commercial electrolyzers won’t be instantaneous. But the research hands developers a clear playbook: minimize interfacial resistance, steer solvation structures, and aim for catalysts that hit the transition potential at lower voltages. Scaling up will involve tackling regulations, ruggedizing equipment, and building partnerships across the supply chain—but with these design rules in hand, those hurdles look more like checkpoints than roadblocks.
The Road Ahead for Green Hydrogen
Good OER catalysts are a big deal for industries from steelmaking to ammonia synthesis. With countries racing toward sustainable energy and industrial decarbonization, cost-effective hydrogen production is set to be a game-changer for feedstocks, energy storage, even fueling zero-emission ships and heavy transport. Reducing the OER energy penalty could shave millions off the price of large-scale electrolyzer systems and ripple benefits across the entire value chain.
Collaboration and Funding Landscape
Europe’s €9 billion Hydrogen Strategy, plus Germany’s national plans, has set the stage for projects like this. Public-private partnerships and multinational consortia are lining up demo projects—think offshore wind-to-hydrogen hubs—that will put these catalysts through their paces at real industrial scale.
Lessons for the Hydrogen Value Chain
Producers, transporters, and end-users stand to gain from catalysts that demand less energy and fewer scarce resources. If new formulations can drop into existing electrolyzer designs with minimal retrofitting, operators could upgrade fleets overnight—slashing downtime and accelerating ROI while advancing green hydrogen infrastructure.
Closing Thoughts
It’s tempting to chase the next flashy catalyst, but sometimes the real magic’s in the tiny gap between catalyst and electrolyte. By homing in on that nanoscale frontier, the Fritz Haber team has charted a bold new path for sustainable energy. If these findings scale up, we might finally see green hydrogen take center stage in our decarbonized future. Stay tuned to upcoming symposia and workshops—your next breakthrough might be a panel conversation away.



 With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.
With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.