Hydrogen Production Reimagined: Membrane-Free Electrolysis Delivers Green H2 and LFP Battery Recycling
September 22, 2025Think you’ve squeezed all you can from electrolyzer design? Think again. On 05/09/2025, a research team upended the playbook by ditching the ion-exchange membrane—long a thorn in the side of cost and complexity—and unveiled a single cell that churns out green hydrogen on demand while plucking lithium straight from spent LFP batteries. It’s hydrogen production and battery recycling wrapped into one sleek package.
Historical Context
For decades, electrolysis has leaned on perfluorinated membranes like Nafion, hiking up CAPEX and spawning constant maintenance headaches. Meanwhile, the boom in LFP packs for EVs and grid storage has leaned on energy-guzzling pyrometallurgy or hydrometallurgy for recycling. Direct electrochemical methods promised a cleaner path but always tripped over selectivity and scale—until now.
Core Innovation
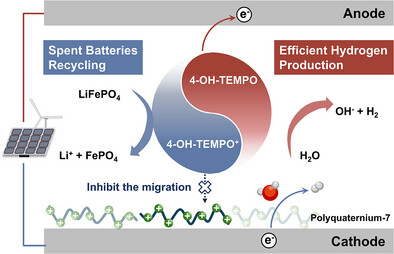
The secret sauce is a clever mash-up of organic chemistry and electrochemical engineering. The team taps 4-HO-TEMPO as a redox mediator to shuttle electrons, and drops in PQ-7, a cationic polyelectrolyte that acts as an electrostatic bouncer—blocking unwanted side reactions. At 100 mA cm⁻², this duo hits a 92.4% faradaic efficiency for hydrogen while pulling lithium from the spent LFP cathode at 54.45 mmol L⁻¹ h⁻¹.
Tech Under the Hood
Traditional cells need membranes to keep gases and ions apart. Rip out that membrane and you save big on cost, but risk cross-contamination. Here, PQ-7 repels the 4-HO-TEMPO molecules from electrode surfaces, sharply cutting parasitic reactions. Meanwhile, Li⁺ ions leave the cathode during oxidation and cruise through the electrolyte for direct harvest. It’s a choreographed dance of water splitting and lithium extraction.
What It Means
Axing the membrane could shave tens of percent off electrolyzer CAPEX and simplify thermal and fluidic design. Combine that with direct battery recycling, and you’re tackling two environmental headaches at once: producing green hydrogen for zero-emission tech and closing the loop on critical minerals without the smelting or acid-bath drama.
Scaling the Promise
Lab results at 100 mA cm⁻² look solid, but industrial electrolyzers often run at 500–1000 mA cm⁻². We need to know how PQ-7 and 4-HO-TEMPO fare after thousands of hours, how polymer fouling is managed, and how they handle a mixed bag of battery scraps. Engineering tweaks, supply-chain clarity, and techno-economic studies are the next steps.
Strategic Angle
If you’re an electrolyzer maker, battery OEM, or recycler, pay attention. Firms like Nel or ITM Power could undercut membrane suppliers, while CATL or Tesla might bake on-site recycling into their factories. With EU and US policies tightening battery recycling quotas and funding sustainable energy and green hydrogen projects, investors will demand pilot-scale proof before writing big checks.
The Maverick Take
I’m impressed, but I’m holding off the confetti. “Membrane-free” doesn’t mean “maintenance-free”—polymers degrade, mediators can foul, and the cost of 4-HO-TEMPO at scale is still an open question. Plus, real-world electrolyzers run hotter and at higher pressures. This chemistry needs to prove it can handle the daily grind with genuine battery waste.
Forward Look
This membrane-free, organic-shielding strategy sketches a circular sustainable energy economy where hydrogen production via electrolysis and battery recycling feed each other. In the coming year, expect pilot demos from national labs or industry consortia. Nail 24/7 operation and mixed-feedstock runs, and you might just see electrolyzers—and the way we manage battery waste—get flipped on their head.
Source: Wiley


 With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.
With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.