Montmorillonite Nanosheet Electrolyte Powers Hydrogen Fuel Cells in Extreme Climates
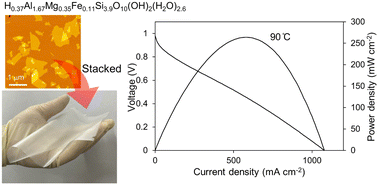
September 25, 2025Over at Kumamoto University, a powerhouse duo—Kazuto Hatakeyama and Shintaro Ida—has just rolled out a game-changing solid electrolyte membrane. What’s the catch? It’s made purely from exfoliated silicate nanosheets sourced from natural montmorillonite clay, with zero binders or polymers in sight. Even better, it shines across a wild temperature range (–10°C to 140°C), delivers proton conductivity of 0.0087 S/cm at 140°C, blocks hydrogen over 100× more effectively than Nafion, and sustains a solid 264 mW/cm² power density at 90°C.
That’s a pretty big deal if you’re tracking fuel cell technology or eyeing the next wave of sustainable energy.
Strategic Implications
Traditional polymer electrolytes like Nafion come with steep price tags, rely on fluorinated stuff, and still let hydrogen sneak through. Ceramic oxides? You need to crank them past 500°C before they even hum. This clay-based film flips the script: it taps into an earth-abundant mineral, slashes material costs, wipes out fluoropolymer headaches, and unlocks cold-climate starts or high-performance automotive applications. If you’re chasing hydrogen fuel cells, industrial decarbonization or green hydrogen solutions in distributed power or mobility, this could be your shortcut to lower total cost of ownership and faster roll-out.
Technology in Brief
At its core are silicate nanosheets with the formula H₀.₃₇Al₁.₆₇Mg₀.₃₅Fe₀.₁₁Si₃.₉O₁₀(OH)₂(H₂O)₂.₆, neatly stacked into a mere 2.5 µm film. Highlights include:
- Proton Conductivity: 0.0087 S/cm at 140°C
- Hydrogen Permeation: >100× lower than Nafion
- Operating Range: –10°C to 140°C
- Power Density: 264 mW/cm² at 90°C
No glue, no plastics—just raw clay nanosheets doing the heavy lifting.
Historical Context
Fuel cells have leaned on either high-temp ceramic oxides (500–1,000°C) or mid-temp fluorinated polymers (~80–90°C) since the 1960s. Each option carries its own trade-offs—cost, leakage or tough operating conditions. Japan’s deep roots in ceramics and clay, especially in Kumamoto Prefecture, made it the perfect launchpad for reimagining montmorillonite as a next-gen electrolyte. This work stands on decades of clay-nanomaterial research, now supercharging the transition to a cleaner grid.
Commercial and Environmental Impact
Turning to a plentiful, low-cost clay could slash membrane costs by up to 50% compared to fluorinated rivals. Better yet, with minimal hydrogen crossover, your cells run safer and more efficiently. The broad temperature window also means you can:
- Fire up automotive fuel cells in frosty mornings
- Keep distributed power units humming under scorching heat
- Deploy grid-scale backups with almost no thermal fuss
And since there’s no fluoropolymer waste, you’re also cutting down on CO₂ emissions in the supply chain—another win for industrial decarbonization and the broader sustainable energy agenda.
Potential Challenges
Of course, scaling up has its own hurdles. You’ll need a reliable pipeline of high-grade montmorillonite and responsible mining practices. Integrating the new membrane with existing electrodes requires fine-tuning the interface to keep resistance low. Plus, real-world durability—think repeated cycling, CO₂ exposure and humidity swings—still needs thorough validation in prototype stacks.
Parallel Trends in Electrolyte Research
Across the globe, teams are tinkering with metal-organic frameworks, phosphoric acid-doped films and hybrid composites. Many struggle to strike the right balance between flexibility, conductivity and gas tightness. The Kumamoto approach stands out by delivering all three in a fully inorganic package, sidestepping polymer degradation and binder headaches.
Key Takeaways
- Montmorillonite breakthrough: flexible, binder-free, 100% inorganic electrolyte.
- Top-tier performance: 0.0087 S/cm at 140°C, 264 mW/cm² at 90°C, >100× better hydrogen barrier.
- Wide operating window: –10°C to 140°C for versatile use cases.
- Cost & eco gains: abundant raw materials, no fluoropolymers, lower environmental footprint.
Looking Ahead
Next up: dialing in the electrode/electrolyte handshake, rolling out large-area membranes and putting them through real-world stack tests. For investors and OEMs in the hydrogen fuel cells arena, this could unlock new markets—from subzero transport to rock-solid backup power—while driving down system costs. Keep an eye on Kumamoto University; they might just have the next big leap in fuel cell technology.
Source: Kumamoto University, Journal of Materials Chemistry A, rsc.org


 With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.
With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.