
Groundbreaking Advancements in Seawater Electrolysis: A New Path for Hydrogen Production
August 16, 2024Researchers Develop Innovative Electrode for Efficient Seawater Electrolysis
In a significant stride towards sustainable energy solutions, researchers from Southern University of Science and Technology in China, University of New South Wales in Australia, and Curtin University in Australia have developed an innovative electrode with the potential to revolutionize hydrogen production. This breakthrough centers on the W-NiFeS/WC electrode, a novel material designed for efficient seawater electrolysis. Let’s dive into the details of this exciting development and understand its implications for the future of clean energy.
Technical Marvel: The W-NiFeS/WC Electrode
At the heart of this advancement is the W-NiFeS/WC electrode. This electrode is composed of self-supported nickel-iron (NiFe) materials that have been enhanced with tungsten (W). The use of wood-based carbon (WC) as a substrate adds another layer of innovation, providing a hierarchical porous structure that significantly boosts the electrode’s performance and stability in seawater.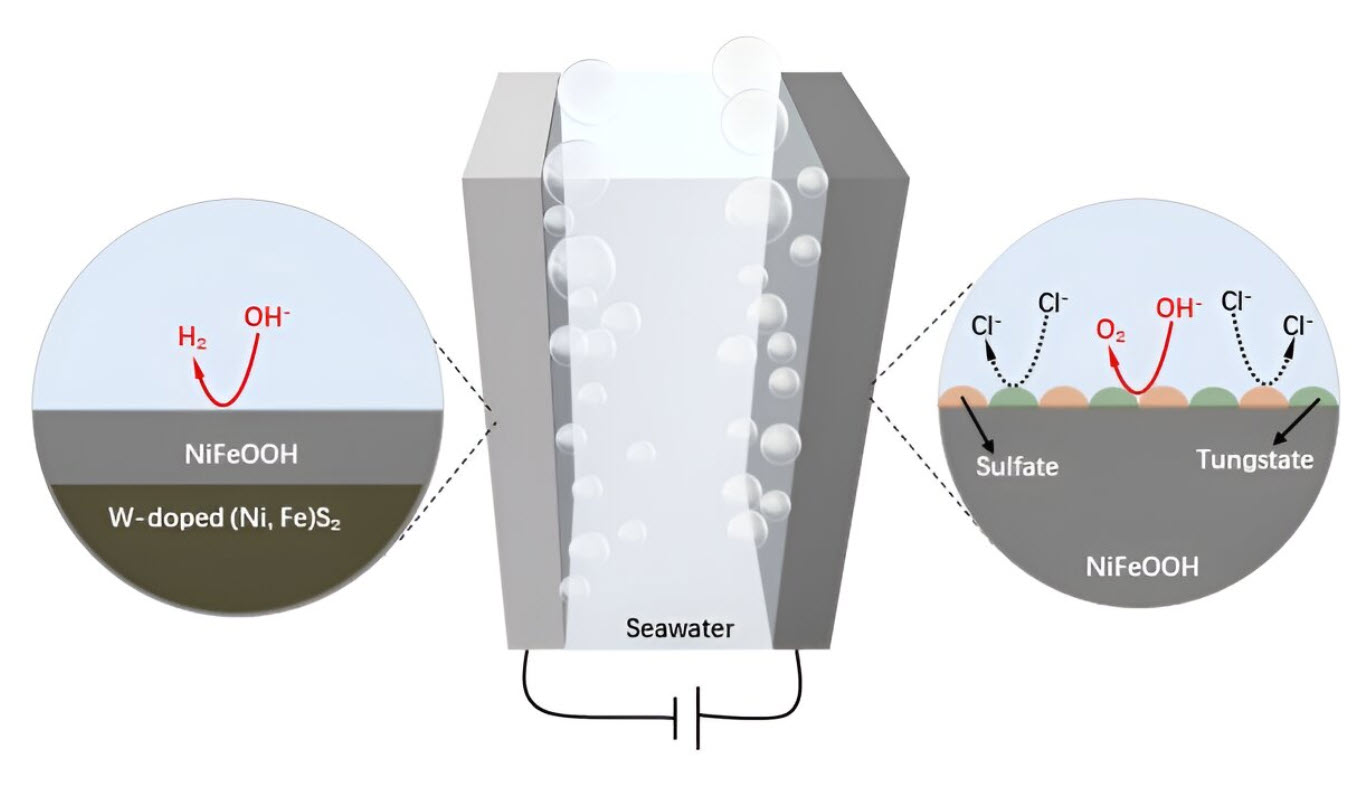
Traditionally, seawater electrolysis has faced significant challenges, including anode corrosion by chloride ions and the high cost of catalysts. The new W-NiFeS/WC electrode addresses these issues head-on. It exhibits a three-dimensional hierarchical porous structure with oriented microchannels and densely anchored W-NiFeS nanoparticles, enhancing its electrical conductivity and efficiency. This design allows it to perform exceptionally well in both the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER), which are crucial for splitting water into hydrogen and oxygen.
Breaking It Down: What Does This Mean?
For those less familiar with electrochemistry, let’s break it down. Electrolysis is a process where electricity is used to split water into hydrogen and oxygen. When we use seawater for this process, it often causes regular electrodes to corrode and wear out quickly because of the salty environment. What the researchers have done is create an electrode that not only withstands these harsh conditions but also performs better than traditional ones.
The electrode’s special structure, with its tiny pores and channels, helps it conduct electricity more efficiently and last longer. This means more hydrogen can be produced over a longer period without the equipment breaking down.
Collaborative Research: A Global Effort
This innovation is the result of a collaborative research effort involving Prof. Hong Chen, Prof. Bing-Jie Ni, and Prof. Zongping Shao from their respective institutions. Their combined expertise has led to the development and fine-tuning of the W-NiFeS/WC electrode through a specialized preparation method involving impregnation and sulfidation. This teamwork underscores the importance of international collaboration in tackling global challenges.
Changing the Game: Sustainable Hydrogen Production
Hydrogen is often hailed as the fuel of the future, but producing it sustainably and efficiently has been a significant hurdle. The development of the W-NiFeS/WC electrode is a game-changer because it allows for the direct use of seawater, an abundant resource, to produce hydrogen. This approach not only makes the process cheaper by eliminating the need for expensive catalysts but also promotes a circular economy by repurposing wood waste into efficient catalysts.
By using this new electrode, we can significantly reduce the environmental impact of hydrogen production, making it a more viable option for widespread adoption. This advancement could lead to cleaner hydrogen fuel that powers everything from electric vehicles to industrial applications, ultimately contributing to the global effort to decarbonize the energy sector.
The Future of Clean Energy
 In summary, the development of the W-NiFeS/WC electrode marks a pivotal moment in the journey towards sustainable hydrogen production. By overcoming the traditional challenges of seawater electrolysis, this innovative technology opens new doors for clean energy solutions. The collaborative efforts of the researchers and the use of waste-derived materials highlight a promising path forward in the quest for a greener planet. Whether you’re an expert in the field or simply interested in the future of clean energy, this breakthrough represents a significant leap towards a more sustainable world.
In summary, the development of the W-NiFeS/WC electrode marks a pivotal moment in the journey towards sustainable hydrogen production. By overcoming the traditional challenges of seawater electrolysis, this innovative technology opens new doors for clean energy solutions. The collaborative efforts of the researchers and the use of waste-derived materials highlight a promising path forward in the quest for a greener planet. Whether you’re an expert in the field or simply interested in the future of clean energy, this breakthrough represents a significant leap towards a more sustainable world.



 With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.
With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.
This sounds like a significant improvement for electrolysis of seawater. I am curious about what happens to all the other elements that are dissolved in seawater when it is split into O2 and H2. When I tried it the other elements in the water precipitated out and formed a sludge that filled the system and shorted it out.