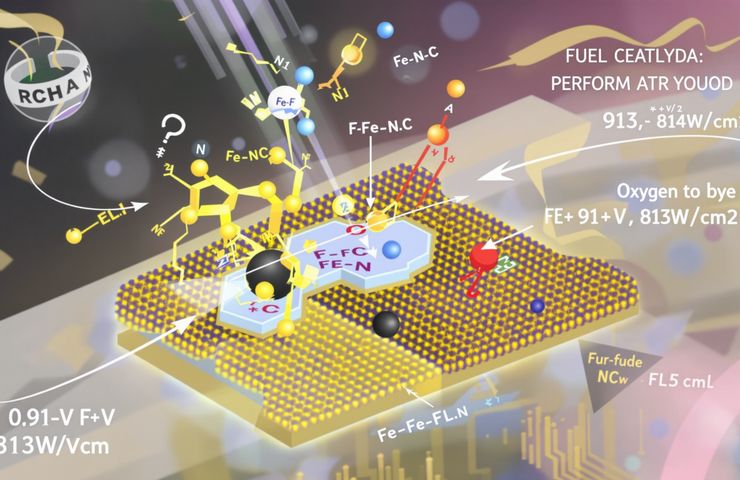
Hydrogen Fuel Cells: New F–Fe–N–C Catalyst Advances Efficiency and Durability
September 5, 2025You’d think platinum is the go-to for top-notch fuel cell performance—but a clever research team decided to shake things up. In their August 20, 2025 paper in Angewandte Chemie, they introduced a game-changing F–Fe–N–C catalyst. The secret? A quasi-covalent Fe–F bond that smartly dodges the usual speed bumps in the oxygen reduction reaction (ORR). The result is pretty impressive: a half-wave potential (E1/2) hitting 0.91 V vs. RHE, and after 80,000 cycles, it barely slipped by 2 mV. Plus, in H2-air tests, it clocked a peak power density of 813 mW/cm². It’s a clear wink toward durable, budget-friendly non-precious metal catalysts for the future of fuel cell technology and hydrogen fuel cells.
This innovation came from a tight-knit crew of electrocatalysts and fuel cell enthusiasts. They cooked up a carbon-based mix, spiking it with iron, nitrogen and fluorine to craft that unique F–Fe–N–C framework. By running rotating disk electrode trials, operando X-ray spectroscopy, and diving into density functional theory models, they proved how the Fe–F bond flexes just right—tuning the grip on key ORR intermediates on the fly.
Historical Context and Research Background
For ages, platinum-group metals (PGMs) have been the rock stars of fuel cell cathodes, driving the ORR—one of the slowest steps in turning hydrogen into electricity. Their performance is unmatched, but their price tag and supply risks have researchers hunting for alternatives. That’s where non-precious metal catalysts like Fe–N–C catalysts come in. They’ve shown promise, but they always bump into the same scaling relationships that limit activity and durability.
In recent years, teams have tried everything—tweaking nitrogen arrangements, adding secondary metals—to chip away at those thermodynamic walls. Still, none have quite cracked the code like this coordination-adaptive trick: embedding a quasi-covalent bond that literally bends the rules in real time.
Parallel Trends in Catalyst Research
This isn’t an iron-only story. Recently, nickel- and cobalt-based catalysts with flexible metal–ligand bonds have popped up, hinting at a broader shift toward dynamic active sites. The goal is the same: let adsorption energies adjust on demand instead of being stuck in one spot. But the F–Fe–N–C catalyst really stands out because it shines not just in rotating disk tests but also in full fuel cell stacks—proof it’s more than lab sleight of hand.
Technical Details
The real magic lies in that dynamic Fe–F bond. Operando spectroscopy, backed by theoretical modeling, showed this bond continuously breaks and reforms during the ORR, fine-tuning how oxygenated intermediates bind. That adaptive coordination lets the catalyst break free from the linear scaling relations that have held conventional Fe–N–C systems hostage.
In rotating disk electrode runs, this tweak paid off big time: E1/2 clocked in at 0.91 V, and after 80,000 stress cycles, performance remained almost untouched. Single-cell trials in anion-exchange membrane fuel cells pushed the envelope further, hitting 813 mW/cm² under H2-air (and even higher with H2–O2), setting a new durability and activity benchmark for non-precious metal catalysts.
Industry Impact
Cost has always been the thorn in fuel cell adoption’s side. Platinum catalysts can gobble up half of a stack’s material bill. By shifting to iron, nitrogen, carbon, and fluorine, this approach could slash costs by more than 70% compared to PGM systems—assuming scale-up goes smoothly. That’s a major boost for anyone tracking sustainable energy trends.
Durability matters just as much as raw performance. Losing only 2 mV after 80,000 cycles is practically unheard of for non-precious setups, and it’s edging close to commercial Pt/C benchmarks. For heavy-duty fleets—where downtime directly hits your bottom line—this resilience could tilt the balance in favor of hydrogen fuel cells over battery electric or diesel options.
Strategic Implications
Major OEMs and scrappy start-ups alike are on the hunt for next-gen catalysts. This coordination-adaptive leap could spark fresh partnerships between academic labs, catalyst producers, and stack integrators. Countries awash in fluorine and iron could position themselves as low-cost suppliers—especially if decarbonization policies incentivize local electrocatalysts manufacturing.
Hydrogen producers have skin in the game, too. Lower catalyst costs and boosted longevity drive down the levelized cost of hydrogen, making green hydrogen even more attractive alongside falling electrolysis expenses. That combo strengthens the pitch for hydrogen in heavy transport, maritime, and grid storage.
Environmental and Economic Benefits
Swapping out PGMs for earth-abundant elements trims the environmental footprint tied to mining and refining scarce metals. Harvesting iron and fluorine tends to be less invasive, and a simpler catalyst recipe should streamline end-of-life recycling.
Plus, widespread use of these affordable non-precious metal catalysts could energize clean energy markets, creating jobs in manufacturing and recycling. That economic ripple, combined with lower emissions, makes this breakthrough a real heavyweight in the move toward sustainable energy.
Looking Ahead
This coordination-adaptive concept opens a fresh gear in electrocatalyst design, pointing to a future where active sites aren’t stuck in one tune. Next steps include scaling production, extended field trials, and integrating these catalysts into commercial stacks. If everything pans out, we’ll likely see the levelized cost of hydrogen fuel cell power drop—and that could be exactly what clean energy needs to break into the mainstream.
Source: Wiley



 With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.
With over 15 years of reporting hydrogen news, we are your premier source for the latest updates and insights in hydrogen and renewable energy.