What is covered on this page…
Part 1 How do hydrogen fuel cells produce electricity
Part 2 What are PEM Fuel Cells
Part 3 H2 gas history
Park 4 Hydrogen Fuel Cell Cars
Part 5 Hydrogen FAQ’s
Part 1 – How do they produce electricity?
Hydrogen fuel cells produce electricity through an electrochemical process.
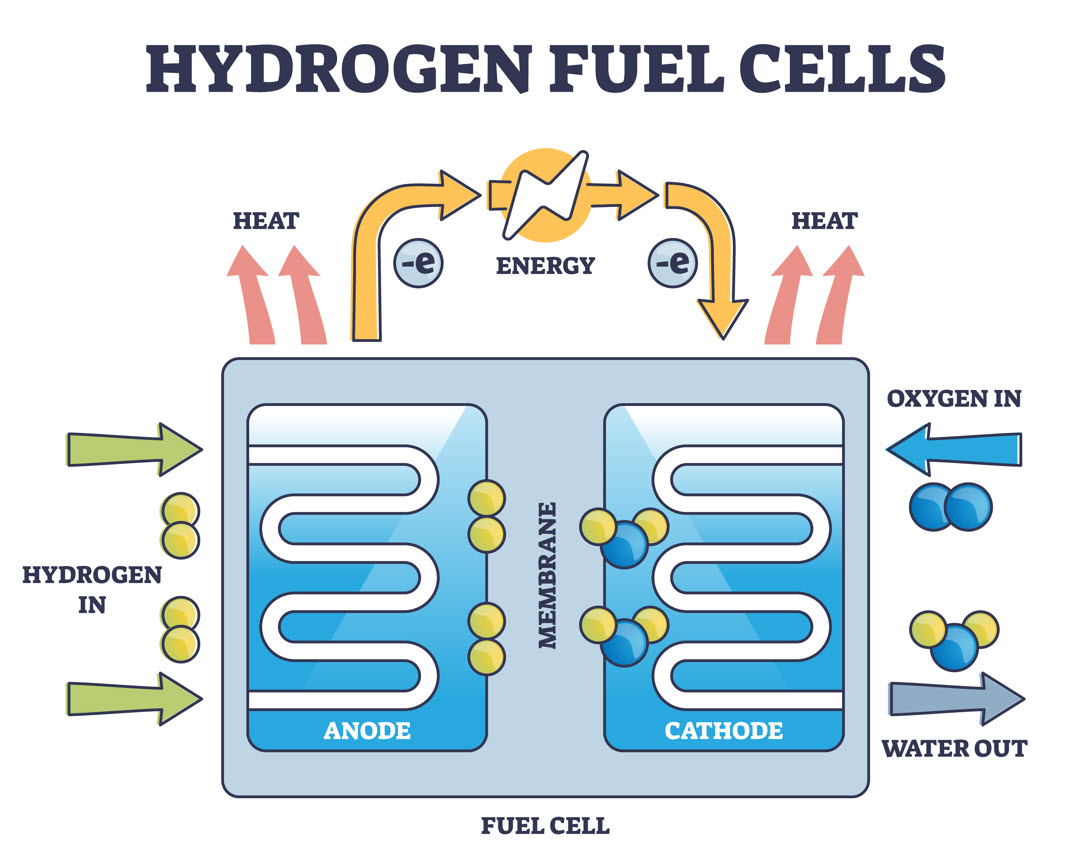
Though there are various types of fuel cells available in the world today, most follow a somewhat straightforward design. Typical fuel cells are made up of three segments: Anode, electrolyte, and cathode.
Two chemical reactions occur within a fuel cell, the results of which are based on the fuel that is used for the energy system. The electrolyte that is used in a fuel cell is highly dependent on the type of fuel cell it is. If hydrogen is used as a fuel, the anode acts as a catalyst that is capable of turning the fuel into electrons and ions.
Next, the cathode converts the ions into the fuel cell’s waste produce, which is typically water vapor, while also using the electrons to generate an electric current.
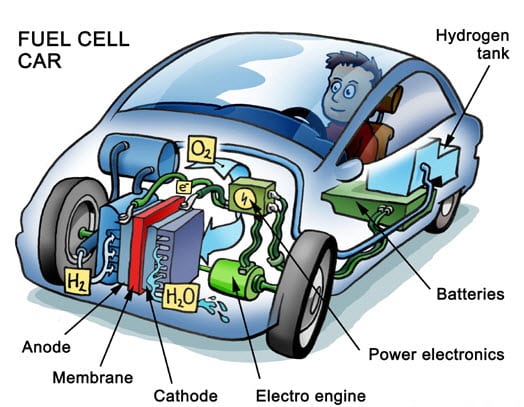
Here we take a look at the process of a fuel cell in a car…
Part 2 – What is PEM Fuel Cells and how do they work?
One of the more popular types of fuel cells are proton exchange membrane (PEM) fuel cells.
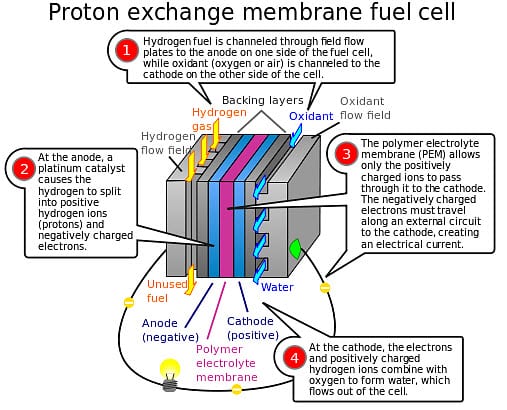
These fuel cells make use of a proton-conducting polymer membrane that acts as an electrolyzer.
This membrane separates the anode and the cathode sides in the fuel cell system. Hydrogen is diffused on the anode side of the fuel cells, producing protons that travel through the membrane to the cathode. The electrons produced by the process are fed into an external circuit, thus creating an electrical charge.
PEM fuel cells are most commonly used by the auto industry when developing hydrogen-powered vehicles.
The fuel cell industry is poised for promising growth, largely due to the support that these energy systems are seeing in many industries. According to the Fuel Cell Today Industry Review 2012, 2011 was one of the most lucrative years the industry has seen, with global shipments growing by 39% over what they had been in 2010. The report suggests that this growth will continue well into the future, meaning that the fuel cell industry may be poised to play a major role in the global energy economy.
Part 3 – Fuel Cell History
Fuel cells are devices that are capable of converting chemical energy acquired through a certain type of fuel into usable electricity. The most common type of fuel used in this process is hydrogen. Hydrogen fuel cells are not a new technology, as some would suggest.
The technology first appeared as a concept in 1838, developed by German scientist Christian Friedrich Schonbein. In 1839, the first fuel cell was publicly demonstration by Welsh scientist Sir William Robert Grove, who based his work on that of Schonbein’s.
Since fuel cells were first developed, they have come to take many forms. Some of these energy systems use methanol as a fuel source to generate electricity, while others use organic waste. The common thread linking all fuel cell systems together is their capability to produce large amounts of clean energy.
Despite their potential to produce large amounts of electricity, fuel cells have been shunned for decades due to technological shortfalls that made them vastly inefficient. Only over the past few years has fuel cell technology reached the point where the energy systems can be considered viable.
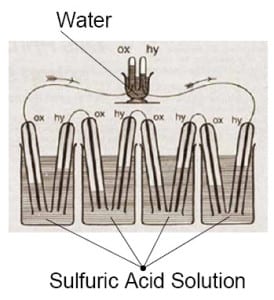 William Groves Fuel Cell Illustration from 1839
William Groves Fuel Cell Illustration from 1839
Part 4 – Hydrogen Fuel Cell Cars and The Automotive Market Today
Hydrogen Fuel Cars Market

Hydrogen Fuel Cells Powering Engines Today
Hydrogen fuel cells have come a long way since their inception. They are no longer limited to being used as industrial power systems; instead, they have found their place in the auto industry, providing clean and efficient energy. Since the 1980s, hydrogen fuel cells have seen a surge in popularity, with most major automakers planning to commercialize hydrogen-powered vehicles equipped with compact yet potent fuel cells.
Among these, Toyota has been a pioneer, particularly with its Mirai model, which is currently one of the top-selling hydrogen cars The Mirai stands as testament to how hydrogen fuel cells can be efficiently utilized in automobiles, offering substantial driving range and quick refueling times.
Furthermore, the interest from the auto industry in hydrogen fuel cells has sparked curiosity in other sectors too. More industries are now considering the adoption of this technology, and it’s also gaining traction as a residential energy system.
Hydrogen Car Market Today
The global auto industry has taken a strong interest in fuel cells. Most of the world’s leading automakers, including Mercedes-Benz, Toyota, Honda, and Hyundai, have already released these vehicles.
Hyundai was the the first to begin mass producing its fuel cell cars, which started to see mass commercialization at the end of 2014. Other automakers started releasing their fuel cell vehicles in 2015 through today.
Companies like Honda and Ford are opting to wait longer to release their fuel cell vehicles due to concerns regarding the capabilities of the hydrogen fuel infrastructure taking form in some countries.
Other Fuel Cell Images…
 This video explains how H2 or Video Hydrogen gas works – From the pump and into a hydrogen fuel car.
This video explains how H2 or Video Hydrogen gas works – From the pump and into a hydrogen fuel car.
Part 5 – FAQs on How Hydrogen Fuel Cells Work

- What is a hydrogen fuel cell? A hydrogen fuel cell is a device that converts chemical energy from a fuel into electricity through an electrochemical reaction of hydrogen fuel with oxygen or another oxidizing agent.
- How do hydrogen fuel cells work? Hydrogen fuel cells work by separating hydrogen into protons and electrons. The protons pass through a membrane, while the electrons create a separate current that can be utilized before they rejoin with the protons and oxygen to produce water.

- What are the main components of a hydrogen fuel cell? The main components of a hydrogen fuel cell are the anode (the positive side), the cathode (the negative side), and the electrolyte (a substance that chemically reacts with the anode and cathode).
- What happens at the anode and cathode in a fuel cell? At the anode, a catalyst causes the hydrogen to split into protons and electrons. The protons pass through the electrolyte to the cathode, while the electrons are forced through a circuit, creating an electric current. At the cathode, the protons, electrons, and oxygen combine to produce water molecules.
- What is the byproduct of a hydrogen fuel cell? The byproduct of a hydrogen fuel cell is water, which makes it an environmentally friendly source of energy.

- Can hydrogen fuel cells be used in cars? Yes, hydrogen fuel cells can be used in cars. In fact, several automakers, including Toyota and Honda, have developed vehicles powered by hydrogen fuel cells.
- How efficient are hydrogen fuel cells? Hydrogen fuel cells are typically around 40-60% energy efficient. However, when the heat they produce is harnessed and used, their efficiency can increase to up to 85%.
- Are hydrogen fuel cells safe? Yes, hydrogen fuel cells are generally safe. However, as with any technology involving flammable materials, there are safety considerations, such as safe handling and storage of hydrogen.
- Do hydrogen fuel cells produce emissions? No, hydrogen fuel cells do not produce harmful emissions. The only byproduct of a hydrogen fuel cell is water vapor, making it a clean energy source.


 HFN News is your leading source for fresh hydrogen and renewable energy updates. Amid the fast-paced growth of hydrogen companies, we provide top-notch news and insights about this exciting sector. Our coverage spans from hydrogen cars to global sustainable initiatives, and we highlight the latest in green jobs and developing hydrogen hubs. We invite you to share your local hydrogen news and explore today’s renewable energy job listings on our site. Thanks for choosing HFN News as your trusted guide to the hydrogen and renewable energy world!
HFN News is your leading source for fresh hydrogen and renewable energy updates. Amid the fast-paced growth of hydrogen companies, we provide top-notch news and insights about this exciting sector. Our coverage spans from hydrogen cars to global sustainable initiatives, and we highlight the latest in green jobs and developing hydrogen hubs. We invite you to share your local hydrogen news and explore today’s renewable energy job listings on our site. Thanks for choosing HFN News as your trusted guide to the hydrogen and renewable energy world!

is it possible and feasible to convert enf existing car from gas to fuel cell . In Britain about 13 years ago i visit in Plymouth south east of britain a shop that was doing the conversion of gas to electric motorisation. Apparently in Britain at that time where charging a pollution tax on new gas car of 10 thousand dollars , but if owner refurbish after 5 years of using gas to electric THEY RECEIVED BACK THAT TAX could get back that pollution tax that where charge to the OFFSET THE NEW ECO CONVERSION .
So if conversion to eco motorization cost 12 thousand s dollars the owner of that new motorization car had only HAD to pay 2 thousand ang got a new car.AND ENVIRONMENT FRIENDLY ON TOP OF THE CHERRY
IS IT POSSIBLE TO HAVE THAT THINKING OF DOING IN NORTH AMERICA
I SAW IT WHIT MY HOW EYES
Excellent article. I would like to get on your mailing list. I now own shares in a company called PLUG that produces fuel cells for lift trucks. The lift trucks are used by many companies in warehouses. To name a few: they are Walmart, Amazon and Home Depot.
I hope this becomes the standard for automobileS.
Would love to know more! How do we
I am trying hard to grasp the principle of fuel cells.
My understanding is that you need a supply of hydrogen as fuel. How is this generated?
Smart move